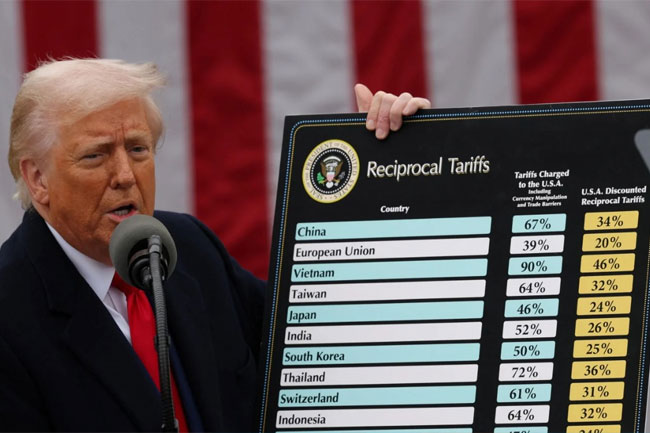
LNP – AKD’s silent, pragmatic leadership enabled SL to reduce U.S. tariffs to 20 per cent – Malik Samarawickrama
Former International Trade Minister Malik Samarawickrama yesterday hailed the government for getting the U.S. reciprocal tariffs reduced to 20 per cent, enabling Sri Lankan goods to be competitive.
Read more